Effects of Lotmaria passim parasites on honeybee behaviour and cognition
Effects of Lotmaria passim parasites on honeybee behaviour and cognition
Background
Honeybees face a wide variety of stressors including habitat loss, climate change, pesticides and parasites that threaten colonies and the vital pollination services they provide. The growing abundance of deadly parasites in apiaries across the world has raised the urgent need to understand the fundamental mechanisms underpinning host/parasite interactions. Withing the main biotic stressors, the trypanosomatid parasite L. passim shows one of the highest prevalence in apiaries worldwide, being capable of reducing honeybee lifespan under experimental conditions as recently demonstrated by our team. This data suggests that L. passim could be a significant threat to colony health, however, there is little information on the fundamental biology of this parasites and their impact on honeybee health.
With the aim of filling this knowledge gap, our overarching goal was to demonstrate that L. passim is also able to interfere in honeybee cognition and behavior at sub-lethal dosages and to describe variations in virulence between different parasite strains. These aims were approached by a research team composed by researchers with experience in molecular parasitology at University of Granada (Spain), bee health at Centro de Investigación Apícola y Agroambiental (Guadalajara, Spain) and bee cognition at Université Paul Sabatier (Toulouse, France) to test this hypothesis. Studying L. passim influence on basic cognitive/behavioural tasks would provide essential knowledge to define the threshold where parasite levels could impact on colony fitness and thus where prophylactic measures would be needed to control trypanosomatid transmission.
Key Findings
To answer the questions raised for this project, we firstly studied the main phenotypic differences found between two different strains (L. passim C1 and C3). isolated from different locations in Andalusia (Spain). As a result of the characterisation of L. passim strains, we unexpectedly found that parasites were able to differentiate into a new parasite form that we defined as trypanosomatid biofilms (Figure 1). This life cycle strategy is one of the most widely distributed and successful modes of life on Earth (bacteria, fungi and algae) but never described before in honeybee parasites.

Figure 1. Scanning electron microscopy of in vitro trypanosomatid biofilms. Image produced at Centro de Instrumentación Científica of the Universidad de Granada (Spain).
Secondly, we compared the performance of two L. passim strains (termed as C1 and C3) in vitro and in vivo. We found that both strains have differences in cell growth but more importantly, their capacity of biofilm formation in vitro was different, indicating that L. passim have different behaviors which could be related to differences in honeybee pathophysiology (Figure 2).

Figure 2. Crystal violet stain for detecting differences between biofilm formation of two different L. passim strains in vitro. The arrow indicates the highest presence of biofilms in L. passim C3 strain compared to C1 strain.
These strains were used to compare their virulence in experimental infections of honeybees. As a result, we found similar trends in both strains, confirming previous results where L. passim induced mortality in infected honeybees and indicating that this is the general trend in nature.
Finally, we tested whether L. passim had any negative effect on honeybee cognition at Behavioural and Cognitive Lab (Tolouse, France) under direction of Prof. Lihoureau. To test this hypothesis, we infected honeybees and trained them in a classical olfactory conditioning of the extension of the proboscis (PER), in which honeybees learn to associate an odour (conditioned stimulus; CS) with a reward of sucrose solution (unconditioned stimulus; US) (Figure 3). The assay was a three trial protocol, consisting in 15 seconds of habituation, 3 sec of CS, 1 second of CS+US, 2 seconds of US, and 29 seconds of clean air, with an intertrial of 10 min. Later, we tested the memory of bees at either 1h (short term memory) or 24h (long term memory) after training, by presenting only the odor. For all the bees that finished the cognitive test, we checked the infection of L. passim in a PCR (Barranco-Gómez et al. 2023).
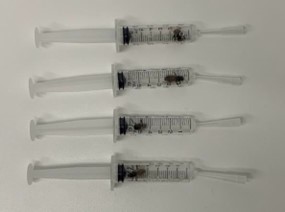
Fig. - Infection Toulouse. Olfactory conditioning of the extension of the proboscis (PER) of infected honeybees with L. passim. Honeybees are slept in ice for 5 min and put inside a syringe where they are provided with a solution of 10 ul of sucrose (20%, w/v) containing the parasite. Bees drinking all the solution are kept in cages of 15 bees at 27ºC for later cognitive experiments.
Nearly 50% of honeybees learnt to associate the odor with the reward, but there were no differences between infected and control honey, suggesting that L. passim does not affect learning in honeybees. In addition, we didn’t find learning in bees infected L. passim in memory neither 1h nor 24h after learning (Figure 3). A higher memory rate was found 1h after training (STM), with nearly 70-75% of bees responding to the odor, while only 25% did it 24 h later (LTM). Altogether, our results suggest that sublethal level of L. passim does not reduce the cognitive abilities in honeybees with the experimental conditions used in this project.
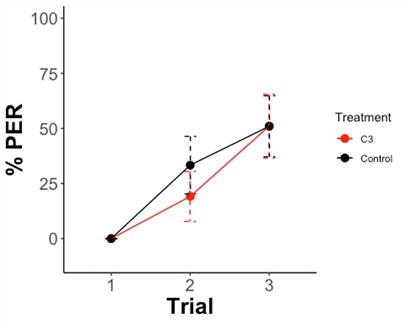
Figure 3. Percentage of PER of bees that responded to the CS during three trials of learning. Sample size (N): C3=33; Control=45. Exposed bees were excluded from the analyses due to low sample size (n=8).
Conclusions and Future Research
The capacity for biofilm formation of these parasites has been one of the main findings of the work. This novel capacity was not expected and was the result of many observations while characterizing L. passim C1 and C3 strains. These interesting results raised several questions such as How these multicellular matrices are formed in the honeybee hindgut? or, How will trypanosomatid biofilms impact the honeybee yeast and bacteria hindgut communities?
On the other side, our research team is currently planning to improve the methodology to find subtle changes in behavioral and cognitive tasks of infected honeybees. We are also about to try new experimental conditions (i.e. infections of adult worker bees, different parasite strains, dosage-response experiments) that could give us some answers to the question on whether or not L. passim is modifying the behavior of infected honeybee colonies.
Thus, we think that funding from the EVA CRANE TRUST has allowed for the discovery of new features of these parasites and raised interesting new questions that will be solved in future work by the consortia formed in the project, combining molecular biological tools, learning and cognitive sciences and bee pathology.
PI: Dr. Mariano Higes Pascual (Marchamalo Beekeeping Research Center (IRIAF-Spain).
Research Team:
- Dr. Luis Miguel de Pablos Torró (University of Granada, Spain).
- Dr. Mathieu Lihoreau (Université Paul Sabatier, Tolouse, France).



